Accueil / Information / Aller plus loin... / Aériens / Pollens / Pollens d’arbres / Les Cupressacées
Les Cupressacées
samedi 20 février 2010, par
Les Cupressacées forment une large famille de plantes appartenant aux Coniférales, ordre où l’on retrouve aussi les Pinacées (les pins). Les Taxodiacées, dont le cèdre du Japon est l’exemple le plus connu, sont à présent rangées au sein des Cupressacées.
Les Cupressacées comprennent, en plus du cèdre du Japon, des arbres dont l’impact allergologique est majeur dans de nombreuses régions : les cyprès et les genévriers, notamment, en ce qui concerne le bassin méditerranéen et le sud des Etats-Unis ![]()
![]()
![]()
![]() .
.
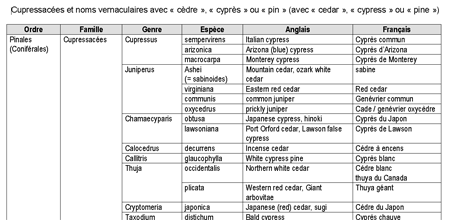
Les pollens des Cupressacées sont plus difficiles à différencier au niveau de l’espèce que pour d’autres familles botaniques et les comptes polliniques agrègent souvent les résultats des différents pollens de Cupressacées.
La terminologie n’améliore pas les choses, car les termes de cèdre (cedar en anglais), cyprès (cypress) ou pin (pine) sont attribués à des noms d’arbres appartenant à des genres, des familles, voire des ordres taxonomiques très différents ! Le tableau ci-après donne un aperçu de ces dénominations.
Dans le midi de la France et en Italie, les Cupressus arizonica (cyprès bleu), Cupressus sempervirens (cyprès vert) et Juniperus oxycedra (cade) sont la cause de la majorité des pollinoses aux Cupressacées.
L’évolution climatique, la pollution ![]()
![]() et des plantations nouvelles sont supposées avoir influencé la progression rapide de la pollinose aux Cupressacées depuis 20-30 ans
et des plantations nouvelles sont supposées avoir influencé la progression rapide de la pollinose aux Cupressacées depuis 20-30 ans ![]()
![]() . D’autres facteurs sont probablement en jeu. Cette évolution concerne aussi le Japon
. D’autres facteurs sont probablement en jeu. Cette évolution concerne aussi le Japon ![]() où la pollinose à Cryptomeria touche environ 15 % de la population.
où la pollinose à Cryptomeria touche environ 15 % de la population.
Dans le midi de la France, une pollinose aux Cupressacées est présente parmi 0,6 à 2,4 % de la population générale ![]() . C’est environ 4 % en Italie. Les prévalences sont plus élevées si l’on retient les TC positifs observés dans une population de sujets polliniques : entre 15 et 35 % en France, Italie, Espagne ou Portugal
. C’est environ 4 % en Italie. Les prévalences sont plus élevées si l’on retient les TC positifs observés dans une population de sujets polliniques : entre 15 et 35 % en France, Italie, Espagne ou Portugal ![]()
![]()
![]()
![]()
![]() .
.
Une étude menée au sein du personnel du CHU de Montpellier a trouvé 15% de TC positifs pour le cyprès ![]() .
.
L’allergénicité de C. arizonica semble supérieure à celle de C. sempervirens ![]()
![]() et le genre Thuja ou bien le cyprès de Leyland sont, à l’inverse, moins allergisants. Dans certaines régions, bien que classées au 3ème rang pour le nombre de pollens émis
et le genre Thuja ou bien le cyprès de Leyland sont, à l’inverse, moins allergisants. Dans certaines régions, bien que classées au 3ème rang pour le nombre de pollens émis ![]() , les Cupressacées y induisent peu ou pas de pollinose : la nature des espèces locales joue donc un rôle important.
, les Cupressacées y induisent peu ou pas de pollinose : la nature des espèces locales joue donc un rôle important.
La pollinose aux Cupressacées se caractérise par une fréquence élevée (entre 10 et 60 %) de patients ayant une pollinose restreinte aux seules Cupressacées ![]()
![]()
![]()
![]()
![]()
![]() .
.
Par ailleurs, ces sujets mono-polliniques se distingueraient par un début de leur pollinose à un âge plus tardif que pour les patients mono-graminées ![]() .
.
Les pollens de Cupressacées contiennent des enzymes à activité de sérine protéase ![]() . Est-ce que cela peut contribuer à leur allergénicité ?
. Est-ce que cela peut contribuer à leur allergénicité ?
Les allergènes des Cupressacées
Si l’allergène Cry j 1 (cèdre du Japon) a été isolé dès 1983 ![]() , soit plusieurs années avant Bet v 1, on est loin de connaître l’ensemble des allergènes des Cupressacées. Par exemple, le blot bidimensionnel du pollen du cèdre du Japon révèle 131 spots différents !
, soit plusieurs années avant Bet v 1, on est loin de connaître l’ensemble des allergènes des Cupressacées. Par exemple, le blot bidimensionnel du pollen du cèdre du Japon révèle 131 spots différents ! ![]()
A l’image des allergènes de graminées ou d’acariens, les allergènes des différentes espèces de cupressacées sont groupés selon les familles protéiques auxquelles ils appartiennent : le groupe 1 des Cupressacées contient des pectate lyases, le groupe 2 des polygalacturonases, le groupe 3 des thaumatine-like, le groupe 4 des protéines liant le calcium (CaBP).
Ces allergènes ont une glycosylation et des séquences peptidiques variables au sein d’un même groupe : on dénombre par exemple 12 isoformes différentes pour Cry j 1 ![]() .
.
Cette variabilité des motifs épitopiques se traduit par une positivité elle-même variable d’un sujet à un autre pour une même isoforme.
Ainsi, la prévalence de positivité pour Cry j 1 a été trouvée entre 27 % et 75 % selon l’isoforme ![]() .
.
- Ces écarts soulignent la forte individualité des réponses immunologiques quand on sait que les isoformes de Cry j 1 sont identiques entre elles à plus de 91 %.
* et les travaux de réactivité croisée entre des produits contenant des formes naturelles multiples d’un même allergène devraient donc être conduits en évitant de pooler (= mélanger) les sérums de plusieurs patients. En effet, cela risque de masquer un résultat positif parmi des résultats négatifs.
De même, l’utilisation d’un clone particulier peut rendre les tests basés sur un recombinant d’interprétation plus limitée.
A ces remarques, valables en dehors des pollens de Cupressacées, s’ajoute le rôle des chaînes glucidiques dont on sait qu’elles peuvent contribuer à une IgE-réactivité croisée sans pertinence clinique prouvée. Et les pollens de Cupressacées sont riches en glycoprotéines.
Le tableau ci-dessous liste les allergènes des groupes 1 à 4 actuellement identifiés :
| Chamaecyparis obtusa | Cyprès du Japon | Cha o 1 | Cha o 2 | ||
| Cryptomeria japonica | Cèdre du Japon | Cry j 1 | Cry j 2 | Cry j 3 | Cry j 4 |
| Cupressus arizonica | Cyprès d’Arizona | Cup a 1 | Cup a 2 | Cup a 3 | Cup a 4 |
| Cupressus sempervirens | Cyprès commun | Cup s 1 | Cup s 3 | ||
| Juniperus ashei | Sabine | Jun a 1 | Jun a 2 | Jun a 3 | |
| Juniperus communis | Genévrier commun | Jun c 1 | |||
| Juniperus oxycedrus | Genévrier oxycèdre | Jun o 1 | Jun o 4 | ||
| Juniperus rigida | Genévrier japonais | Jun r 3 | |||
| Juniperus virginiana | Red cedar | Jun v 1 | Jun v 3 | Jun v 4 | |
| Taxodium distichum | Cyprès chauve | Tax d 2 | |||
| Thuja occidentalis | Thuya du Canada | Thu oc 3 | |||
| Thuja plicata | Thuya géant | Thu p 1 |
Les allergènes du groupe 1
On a caractérisé plusieurs allergènes ayant une structure les apparentant aux pectate lyases, même si l’activité enzymatique de ces allergènes est parfois négligeable ou déficiente ![]() :
:
- Cry j 1 (cèdre du Japon),
- Cha o 1 (cyprès du Japon),
- Cup a 1 (cyprès de l’Arizona),
- Jun a 1 et
- Jun v 1 (genévriers), etc…
L’homologie entre ces allergènes est forte (75 à 91 %). Elle est par contre limitée avec des pectate lyases d’autres pollens (45-49 % avec Amb a 1 ou Amb a 2 de l’ambroisie) ou avec des pectate lyases contenues dans des aliments végétaux (ex : fraise) ![]()
![]()
![]()
![]()
![]()
![]() .
.
La positivité in vitro pour Cry j 1, Cup a 1 ou Jun a 1 est comprise entre 75 et 86 % des sujets exposés aux pollens correspondants ![]()
![]()
![]()
![]() .
.
Des sujets non exposés peuvent aussi présenter un résultat positif du fait de la réactivité croisée entre ces allergènes : cela est clair quand les arbres sont absents de l’environnement des patients, comme le cèdre du Japon en France ou aux USA et la sabine au Japon.
- Ainsi 40 % des polliniques à Juniperus ashei (USA) étaient trouvés positifs pour Cry j 1 (cèdre du Japon)
 .
. - Et inversement 75 % de Japonais polliniques à Cryptomeria étaient positifs pout Jun a 1
 .
.
Les études de positionnement des épitopes B sur Jun a 1 et Cry j 1 ont montré l’existence d’épitopes séquentiels et d’épitopes conformationnels ![]() .
.
- Dans l’ensemble, l’homologie entre allergènes du groupe 1 est bonne au niveau du site enzymatique
 , mais des différences existent suscitant une réactivité croisée non systématique
, mais des différences existent suscitant une réactivité croisée non systématique  .
. - Ainsi une séquence de Cry j 1 est 100 % identique avec la zone équivalente sur Jun a 1 : mais si cette dernière est un épitope pour Jun a 1, ce n’est pas un épitope sur Cry j 1
 .
. - Des paramètres conformationnels doivent entrer en ligne de compte. Cela peut résulter de différences au niveau des glycosylations entre ces 2 allergènes
 .
.
Les allergènes du groupe 2
Ils ont été moins étudiés que ceux du groupe 1 ![]()
![]()
![]() , même si ce sont des allergènes très souvent positifs in vitro (ex : 70 % pour Cry j 2
, même si ce sont des allergènes très souvent positifs in vitro (ex : 70 % pour Cry j 2 ![]()
![]() ). Ils ont une bonne homologie entre eux : entre 71 % et 82 % d’identité entre Cry j 2, Jun a 2 et Cha o 2
). Ils ont une bonne homologie entre eux : entre 71 % et 82 % d’identité entre Cry j 2, Jun a 2 et Cha o 2 ![]() .
.
Ces allergènes sont des polygalacturonases.
L’activité enzymatique de ces allergènes est un peu différente de celle des polygalacturonases d’autres pollens (ex. graminées) ou d’aliments végétaux : Cry j 2 a une activité polymethyl-galacturonase.
La réactivité croisée des allergènes du groupe 2 semble limitée aux seuls pollens de Cupressacées entre eux ![]() . Et si l’on a pu montrer des cDNA homologues dans la plupart des familles de Coniférales, ce type de protéine ne semble pas être exprimé dans les Pinacées
. Et si l’on a pu montrer des cDNA homologues dans la plupart des familles de Coniférales, ce type de protéine ne semble pas être exprimé dans les Pinacées ![]() .
.
Les allergènes du groupe 2 sont le plus souvent positifs en même temps que ceux du groupe 1. On trouve cependant plus de sujets mono-positifs groupe 1 que groupe 2. Et cette différence se retrouve dans la réactivité in vitro souvent plus nette pour Cry j 1 ![]() .
.
Les allergènes du groupe 3
Ce sont des thaumatine-like. Cette famille de protéines de défense végétale comprend, entre autres, Cap a 1 (poivron), Pru av 2 (cerise) et Mal d 2 (pomme).
Les possibilités de réactivité croisée entre pollens de Cupressacées et aliments sont limitées par la faible identité entre les thaumatine-like de ces produits :
Par contre, les pourcentages d’identité entre allergènes du groupe 3 sont élevés parmi les Cupressacées :
- jusqu’à 85-95 % entre Cup a 3, Jun a 3, Cry j 3, Cup s 3, Jun r 3 (Juniperus rigida), ou Thu oc 3 (Thuja occidentalis)



 .
.
Comme pour ceux du groupe 1, les allergènes du groupe 3 se présentent sous différentes isoformes ![]() . Et si l’homologie au niveau épitopique n’est pas toujours optimale
. Et si l’homologie au niveau épitopique n’est pas toujours optimale ![]() , une réactivité croisée entre allergènes du groupe 3 a été montrée
, une réactivité croisée entre allergènes du groupe 3 a été montrée ![]() .
.
Toutes les thaumatine-like ne sont pas glycosylées. En ce qui concerne les allergènes du groupe 3, une glycosylation a été montrée au moins pour Cry j 3 et Jun a 3 ![]() .
.
Autres allergènes
Une profiline dans le pollen de cyprès Cupressus sempervirens ![]() .
.
Une protéine liant le calcium, Jun o 4, dans le pollen de cade (Juniperus oxycedra) ![]() :
:
- Cet allergène, autrefois dénommé Jun o 2, a la particularité de posséder 4 sites de liaisons pour le calcium (c’est donc une « 4 EF-hand »), contrairement aux polcalcines rencontrées dans les pollens de Bétulacées, Graminées, etc… qui sont des « 2 EF-hand ».
- On trouve aussi des « 4 EF-hand » dans le cèdre du Japon (Cry j 4), le cyprès d’Arizona (Cup a 4) et le red cedar (Jun v 4). L’identité entre ces protéines et leur homologue dans l’olivier, Ole e 8, est faible
 .
.
Une isoflavone réductase a été montrée IgE-réactive dans le pollen du cèdre du Japon.
- Elle est homologue de Bet v 6 (bouleau) et Pyr c 5 (poire), les niveaux d’identité (environ 60 %) ne permettant pas de conclure à une réactivité croisée potentielle ou non.
- Cette isoflavone réductase serait IgE-réactive chez 75-80 % des patients polliniques au cèdre du Japon

 .
.
Une chitinase de classe 4 a aussi été notée IgE-réactive chez plus de 50 % des polliniques au cèdre du Japon ![]() . Ce type de chitinase possédant un domaine hévéine, il était intéressant de quantifier les % d’identité de différents domaines hévéine avec celui de cet allergène :
. Ce type de chitinase possédant un domaine hévéine, il était intéressant de quantifier les % d’identité de différents domaines hévéine avec celui de cet allergène :
- pour l’hévéine du latex (Hev b 6.02) il a été trouvé 39 %
- entre 44 et 47 % pour les domaines hévéine des chitinases de classe 1 dans l’avocat, la banane ou la châtaigne Les taux sont relativement faibles et sont moyennement favorables à une réactivité croisée
- s’agissant des domaines hévéine des chitinases de classe 4, cette fois, les % d’identité sont plus élevés (ex : 65 % pour la chitinase classe 4 du raisin). Mais l’existence d’une réactivité croisée cèdre du Japon-raisin ou colza ou haricot, par exemple, n’a pas été étudiée.
Une réactivité en blot à 46 et 50 kDa a été montrée avec les pollens de cyprès commun et de cyprès d’Arizona ![]() . Les fractions présentent une activité béta-galactosidase.
. Les fractions présentent une activité béta-galactosidase.
Une LTP est présente dans le pollen de cyprès d’Arizona ![]() . Son IgE-réactivité est à confirmer.
. Son IgE-réactivité est à confirmer.
Une extraction poussée des protéines du pollen de cyprès commun a permis de mettre en évidence en immunoblot une réactivité supplémentaire vis à vis d’une protéine basique de 10-14 kDa, non encore identifiée ![]() .
.
Par contre, l’existence de polcalcines IgE-réactives est suggérée par une étude en tests cutanés où, sur les 16 patients positifs pour une polcalcine purifiée de palmier (Phoenix dactylifera), 14 étaient positifs pour le pollen de cyprès ![]() .
.
Réactions croisées entre pollens de Cupressacées
De nombreuses études ont montré que les pollens de Cupressacées pouvaient croiser entre eux. Il faut cependant souligner que l’origine des patients influe sur la réactivité croisée : le pollen local inhibe mieux un pollen exotique que l’inverse. Par exemple le cyprès commun inhibe le cèdre du Japon chez des patients français mais pas chez des patients japonais ![]() .
.
Le croisement entre allergènes de Cupressacées peut s’opérer aussi au niveau des cellules T : c’est le cas pour Cry j 1 et Cha o 1. Cela pourrait expliquer, avec l’aide d’autres pectate lyases (ex : banane ou Amb a 1 d’ambroisie), le maintien perannuel de cellules mémoires spécifiques à Cry j 1 ![]()
![]()
![]() . En ce qui concerne le Japon, on pourrait aussi ajouter la persistance indoor des pollens de cèdre du Japon bien après la saison pollinique
. En ce qui concerne le Japon, on pourrait aussi ajouter la persistance indoor des pollens de cèdre du Japon bien après la saison pollinique ![]() .
.
Réactions croisées entre Cupressacées et d’autres pollens
Des résultats positifs (et inconstants) de réactivité croisée ont été montrés entre pollens de Cupressacées et ceux de graminées, ambroisie, chénopode, pariétaire, plantain, etc…
Le support de ces réactions croisées est mal défini.
Des profilines ? Cela est possible mais l’on n’a caractérisé une profiline IgE-réactive que dans le cyprès commun pour le moment.
Ces réactions croisées entre pollens pourraient correspondre à des polcalcines.
- Celle d’aulne inhibe Jun o 4 du pollen de cade (mais pas l’inverse)
 .
. - Mais les preuves d’une réactivité croisée entre « 2EF-hand » et « 4EF-hand » sont faibles.
Dans le cas de l’olivier, une réactivité croisée a été montrée pour certains patients avec le cyprès commun ou le cyprès blanc ![]()
![]()
![]()
![]() .
.
- Et dans un travail, pour le moment isolé, une fraction à activité béta-galactosidase croisait entre olivier et cyprès

- Les polcalcines Jun o 4 et Ole e 8, toutes deux « 4EF », n’ont cependant que 27 % d’identité entre elles
 .
.
In vitro, les réactions croisées peuvent aussi avoir pour support des CCD. Ces épitopes glucidiques croisants sont courants dans tous les pollens.
Au total, le développement d’une pollinose aux Cupressacées par des pollens d’autres origines (ou l’inverse) n’est pas prouvé.
Au contraire, le pourcentage relativement élevé de mono-réactivité aux pollens de Cupressacées évoque un pouvoir sensibilisant autonome pour ces pollens.
Cupressacées et allergie alimentaire
Au Japon, un travail il a été montré une réaction croisée entre tomate et cèdre du Japon ![]() . L’allergie à la tomate a été vérifiée par TPO dans un autre travail
. L’allergie à la tomate a été vérifiée par TPO dans un autre travail ![]() .
.
De même, au Texas, une équipe a montré une inhibition de la tomate par le genévrier ![]() .
.
La même réaction croisée avec le genévrier était vue pour la pomme et la banane ![]() , des aliments au total assez différents sur le plan des allergènes.
, des aliments au total assez différents sur le plan des allergènes.
Ces associations pourraient provenir de thaumatine-like (Cry j 3, Jun a 3, Mal d 2 pomme, protéine P23 tomate, etc…). Des études bio-informatiques ont en effet montré de possibles communautés épitopiques entre ces protéines ![]()
![]() . Et il a été trouvé 78% de positifs pour une thaumatine-like de l’acarien Glycyphagus parmi des patients italiens polliniques, contre 12% parmi des allergiques aux acariens vivant à Singapour
. Et il a été trouvé 78% de positifs pour une thaumatine-like de l’acarien Glycyphagus parmi des patients italiens polliniques, contre 12% parmi des allergiques aux acariens vivant à Singapour ![]() , avec l’hypothèse suivante : cette thaumatine-like (66% d’identité avec Mal d 2) est reconnue par des patients sensibilisés à des allergènes homologues de Cupressacées.
, avec l’hypothèse suivante : cette thaumatine-like (66% d’identité avec Mal d 2) est reconnue par des patients sensibilisés à des allergènes homologues de Cupressacées.
Cependant, une faible pertinence pour ces éventuelles « allergies croisées » est suggérée par deux études japonaises :
- dans une étude, les patients polliniques au cèdre du Japon (ce qui est peu sélectif dans ce pays) et présentant un syndrome oral, avaient également des CAP bouleau positifs en grand excès par rapport à la clinique
![- Ishida T, Murai K, Yasuda T, Satou T, Sejima T, Kitamura K. [Oral allergy syndrome in patients with Japanese cedar pollinosis]. Nippon Jibiinkoka Gakkai Kaiho 2000;103:199-205](plugins/allerdata/theme/images/book_open.png) , ce qui oriente vers un panallergène (profiline ?) et n’est pas en faveur d’un lien spécifique cèdre-aliment
, ce qui oriente vers un panallergène (profiline ?) et n’est pas en faveur d’un lien spécifique cèdre-aliment - et dans l’autre étude les prévalences pour un syndrome oral n’étaient pas plus élevées chez des polliniques au cèdre que chez des allergiques aux acariens
![- Arai Y, Ogawa C, Ohtomo M, Sano Y, Ito K. [Food and food additives hypersensitivity in adult asthmatics. II. Oral allergy syndrome in adult asthmatic with or without Japanese cedar hay fever]. Arerugi 1998;47:715-719](plugins/allerdata/theme/images/book_open.png) .
.
En France, des travaux sont en cours pour déterminer la réalité immunologique d’une association Cupressacées-pêche (cf. pêche et cyprès).
Pollens de Cupressacées et latex
Un travail montre que le latex est capable d’inhiber la réactivité à la chitinase de classe 4 du cèdre du Japon ![]() .
.
Il est possible que l’hévéine du latex soit le support de cette réaction croisée, mais les pourcentages d’homologie sont peu favorables et la vérification d’une réactivité croisée avec l’hévéine plutôt que l’extrait latex n’a pas été tentée.
D’autres études sont nécessaires avant de pouvoir parler d’ « allergie croisée » latex-cèdre du Japon.
Diagnostic d’une sensibilisation aux Cupressacées
Le diagnostic d’une pollinose aux Cupressacées a longtemps été basé sur des critères cliniques et chronologiques avant tout, car l’extraction des protéines de ces pollens est difficile (peu de protéines, beaucoup de glucides, des pigments).
Des progrès dans les techniques d’obtention des extraits ont permis d’avoir un diagnostic plus précis.
Malgré tout, la présence de différentes espèces dans la même région, les périodes de pollinisation qui se chevauchent et/ou se succèdent et la fréquence des réactions croisées entre pollens de Cupressacées rend parfois délicat le diagnostic d’une pollinose à une espèce précise plutôt qu’une autre.
Des tests in vitro utilisant des allergènes purifiés du groupe 1 sont envisagés ![]() . Dans l’étude EXPO couvrant le territoire espagnol, la sensibilité d’un test avec nCup s 1 était de 65% comparativement aux tests cutanés
. Dans l’étude EXPO couvrant le territoire espagnol, la sensibilité d’un test avec nCup s 1 était de 65% comparativement aux tests cutanés ![]() . Il faudra cependant tenir compte d’une réactivité de type CCD.
. Il faudra cependant tenir compte d’une réactivité de type CCD.
Pollens de Cupressacées et CCD
(voir aussi : Les CCD)
Plusieurs allergènes de Cupressacées sont glycosylés.
Une réactivité de type CCD est donc prévisible.
Elle a été vérifiée de diverses façons :
- chute de l’IgE-réactivité après traitement de l’extrait (ou de l’allergène) par du periodate

 , ou par des glyco-reporters comme la broméline et l’HRP
, ou par des glyco-reporters comme la broméline et l’HRP  ,
, - inhibition de la broméline ou de la PLA2 d’abeille (Api m 1) par un extrait de cyprès
 ,
, - réactivité avec l’allergène naturel nCup a 1 mais pas avec le recombinant non glycosylé rCup a 1

 ,
, - inhibition de nCup a 1 par le glycopeptide de broméline MUXF

- résultats plus élevés en CAP qu’avec la technique Centaur (moins affectée par les CCD
 ) chez des sujets non exposés aux pollens de Cupressacées
) chez des sujets non exposés aux pollens de Cupressacées  .
.
Les avis sont cependant partagés sur la part de la réactivité glucidique de l’allergène Cry j 1 (cèdre du Japon) :
- importante
 ou faible
ou faible  .
. - Pour di Felice
 la glycosylation aurait un rôle indirect : celui de favoriser une structure 3D correcte et, de là, la réactivité des épitopes conformationnels.
la glycosylation aurait un rôle indirect : celui de favoriser une structure 3D correcte et, de là, la réactivité des épitopes conformationnels.
Il est possible que Cry j 1 ait aussi une réactivité glucidique plus faible que d’autres allergènes du groupe 1 du fait du type des chaînes glucidiques :
- Cha o 1 (cyprès du Japon) possède majoritairement des chaînes GnGnXF (89%)
 , de même que Cup a 1 (cyprès d’Arizona)
, de même que Cup a 1 (cyprès d’Arizona)  et Jun a 1 (sabine)
et Jun a 1 (sabine) 
- Cry j 1 possède moins de chaînes GnGnXF (47 %) et plus de chaînes Lewis a (53%)

 . Ces dernières sont données pour peu IgE-réactives
. Ces dernières sont données pour peu IgE-réactives  .
.
Cependant la réactivité de type CCD du Cup a 1 est malgré tout plus faible que celle d’allergène glycosylés d’autres pollens : dans l’étude EXPO ![]() , les régions de fortes prévalence de réactivité pour les graminées (l’ouest) ou pour l’olivier (le sud) présentaient des taux de positivité pour nCup a a1 (glycosylé) somme toute assez modérés.
, les régions de fortes prévalence de réactivité pour les graminées (l’ouest) ou pour l’olivier (le sud) présentaient des taux de positivité pour nCup a a1 (glycosylé) somme toute assez modérés.
Une histamino-libération a pu être montrée avec nCup a 1 chez des sujets réagissant à des glyco-épitopes car négatifs in vitro pour rCup a 1 (non glycosylé) ![]() . Ce type de résultats expérimentaux est le principal argument en faveur d’une possible relevance clinique des épitopes glucidiques (cf. Les CCD).
. Ce type de résultats expérimentaux est le principal argument en faveur d’une possible relevance clinique des épitopes glucidiques (cf. Les CCD).
Par ailleurs, il a été montré que Cry j 1 pouvait stimuler des réponses Th2 également par sa glycosylation ![]() .
.