Accueil / Information / Aller plus loin... / Aériens / Pollens / Pollens de graminées / Les graminées
Les graminées
lundi 1er mars 2010, par
Les graminées, ou Poacées, représentent environ le quart de la surface végétale du globe. Elles sont présentes dans une grande variété de climats et sont une cause majeure de pollinose.
Cependant, la plupart des études allergologiques et moléculaires sur les graminées ont concerné un nombre très réduit d’espèces (20 à 30 sur les quelque 10 000), notamment d’espèces croissant en climat tempéré.
Il est certain que cette exploration constamment ciblée sur les mêmes espèces, par exemple la fléole, ne donne pas un reflet correct de la diversité des pollens de graminées présents dans l’environnement du patient et donc des pollens effectivement responsables de ses symptômes.
Ce pourrait être le cas pour le chiendent digité (Cynodon dactylon), dont l’aire de présence tend à s’étendre vers des régions non tropicales et qui croise pas ou peu avec les graminées tempérées classiques.
Le tableau ci-dessous montre l’arbre taxonomique de quelques Poacées et leur dénomination anglaise habituelle (en italiques : le nom est suivi de "grass") :
| genre | anglais | français | |||
|---|---|---|---|---|---|
| BEP clade | Bambusoïdées | Bambusées | bamboos | bambous | |
| ^ | Ehrhartoïdées | Oryzées | Oryza | rice | riz |
| Zizania | wild rice | riz sauvage | |||
| Pooïdées | Avénées | Agrostis | redtop | agrostide | |
| Alopecurus | foxtail | vulpin | |||
| Anthoxanthum | sweet vernal | flouve | |||
| Avena | oat | avoine | |||
| Holcus | velvet | houlque | |||
| Koeleria | june | koélérie | |||
| Phalaris | canary | alpiste | |||
| Phleum | timothy | fléole | |||
| Trisetum | oatgrass | trisète | |||
| Bromées | Bromus | brome | brome | ||
| Poées | Dactylis | orchard | dactyle | ||
| Festuca | fescue | fétuque | |||
| Lolium | ryegrass | ivraie | |||
| Poa | kentucky meadow |
pâturin | |||
| Triticées | Agropyron | quack | chiendent | ||
| Hordeum | barley | orge | |||
| Secale | rye | seigle | |||
| Triticum | wheat | blé | |||
| PACCAD clade | Aristidoïdées | Aristidées | Aristida | three awn | |
| Arundinoïdées | Arunidinées | Arundo | giant reed | roseau | |
| Cordateria | pampas | herbe pampa | |||
| Phragmites | common reed | roseau | |||
| Chloridoïdées | Cynodontées | Bouteloua | grama | ||
| Cynodon | bermuda | chiendent digité | |||
| Distichlis | salt | herbe prés salés | |||
| Eragrostis | love | éragrostide | |||
| Sporolobus | dropseed | ||||
| Panicoïdées | Andropogonées | Imperata | cogon | alang alang | |
| Saccharum | sugarcane | canne à sucre | |||
| Sorghum | johnson | sorgho | |||
| Zea | corn | maïs | |||
| Panicées | Cenchrus | english bunch | |||
| Digitaria | crab | ||||
| Panicum | millet | millet | |||
| Paspalum | bahia | herbe bahia | |||
| Pennisetum | kikuyu | ||||
| Stenotaphrum | buffalo |
La position de telle ou telle graminée dans l’arbre taxonomique donne une indication sur l’éloignement moléculaire des allergènes et donc sur la facilité ou non qu’ont ces allergènes à croiser entre eux.
Par exemple, les pollens rencontrés en Inde (Cynodon, Pennisetum, Imperata, Cenchrus, Sorghum) ![]()
![]()
![]()
![]() , dans le sud des USA (Paspalum, Cynodon, Sorghum)
, dans le sud des USA (Paspalum, Cynodon, Sorghum) ![]()
![]() , ou en Afrique du Sud (Pennisetum, Aristida, Stenotaphrum, Eragrostis)
, ou en Afrique du Sud (Pennisetum, Aristida, Stenotaphrum, Eragrostis) ![]()
![]() appartiennent au clade PACCAD des graminées et croisent peu avec les graminées tempérées
appartiennent au clade PACCAD des graminées et croisent peu avec les graminées tempérées ![]() (cf. réactivités croisées entre pollens de Graminées.
(cf. réactivités croisées entre pollens de Graminées.
Mais d’autres pollens rencontrés dans des climats volontiers chauds sont cross-réactifs : Lolium multiflorum au Brésil ![]() , Phalaris aquatica en Australie
, Phalaris aquatica en Australie ![]() ou Trisetum paniceum en Espagne centrale
ou Trisetum paniceum en Espagne centrale ![]()
![]() . Ces pollens se trouvent dans des sous-familles (Poeae) ou des tribus (Aveneae) de Graminées tempérées classiques.
. Ces pollens se trouvent dans des sous-familles (Poeae) ou des tribus (Aveneae) de Graminées tempérées classiques.
Les pollens de graminées sont d’un diamètre relativement important (20-55 μ) mais ils donnent naissance à des granules cytoplasmiques de faible dimension (≤ 2,5 μ) dès qu’ils s’hydratent suffisamment. Ces fragments sont suspectés être la cause des asthmes épidémiques après orage ![]()
![]() .
.
La répartition des allergènes dans les granules est un peu différente de celle du pollen entier, même si les allergènes principaux y sont représentés (groupes 1, 2, 5 et 6) ![]()
Pollinose aux Graminées
La prévalence de la pollinose aux graminées est estimée à 40-70 % parmi les sujets polliniques ![]()
![]()
![]() . Un TC positif pour un mélange de graminées a été trouvé chez 23 à 50 % des sujets testés dans le protocole GA²LEN
. Un TC positif pour un mélange de graminées a été trouvé chez 23 à 50 % des sujets testés dans le protocole GA²LEN ![]() .
.
Une réactivité pour les graminées, sans autre positivité pour d’autres pollens, est rencontrée dans une proportion variable de patients, selon les conditions de sélection des sujets étudiés. Mari rapporte 13 à 18 % de patients mono-graminées ![]()
![]() mais des taux plus importants ont été donnés par d’autres auteurs (30-45 %)
mais des taux plus importants ont été donnés par d’autres auteurs (30-45 %) ![]()
![]() .
.
La sensibilisation aux allergènes de graminées dépend de facteurs environnementaux mais aussi individuels.
Une composante génétique favorise la sensibilisation à tel allergène et même à tel épitope sur un allergène donné ![]() .
.
Des profils individuels très variés sont visibles ![]() et semblent relativement stables dans le temps chez le même sujet
et semblent relativement stables dans le temps chez le même sujet ![]()
![]() . Ainsi, l’apparition de néo-réactivités IgE au cours d’une immunothérapie n’est vue que chez une minorité de patients
. Ainsi, l’apparition de néo-réactivités IgE au cours d’une immunothérapie n’est vue que chez une minorité de patients ![]()
![]()
![]() .
.
Les graminées céréalières sont globalement moins responsables de pollinose, au moins isolément : il est rare d’observer une positivité pour une graminée céréalière sans positivité simultanée pour une graminée fourragère. Les céréales semblant les plus associées à une pollinose aux graminées sont le seigle et l’orge ![]() .
.
Le pollen de maïs a une allergénicité considérée comme faible. Des cas isolés de pollinose au maïs ont été décrits ![]() .
.
Une enquête française montre pourtant un taux de "sensibilisation" non négligeable pour ce pollen, surtout en zone de forte culture de maïs (environ 15 %) ![]() . La faible allergénicité du pollen de maïs est attribuée à sa taille (85-125 μ) rendant les grains trop lourds pour être facilement dispersibles au loin
. La faible allergénicité du pollen de maïs est attribuée à sa taille (85-125 μ) rendant les grains trop lourds pour être facilement dispersibles au loin ![]() .
.
Les allergènes de pollens de graminées
La forte prévalence de l’allergie aux pollens de graminées a conduit à une étude très poussée de leurs allergènes. Comme pour les acariens, on distingue des "groupes" d’allergènes. Pour le moment ces groupes vont du groupe 1 au groupe 13.
La relevance clinique des différents groupes n’a pas été étudiée en soi car il est rare de trouver des patients monosensibilisés à tel ou tel groupe. L’opinion prévaut que les groupes 1 et 5 sont cliniquement importants ![]() , tandis que les groupes 7 et 12 représentent la trace d’une poly-réactivité pollinique sans incidence clinique en règle générale.
, tandis que les groupes 7 et 12 représentent la trace d’une poly-réactivité pollinique sans incidence clinique en règle générale.
Il arrive parfois cependant qu’une sensibilisation aux profilines puisse générer une symptomatologie respiratoire : tel ce patient pollinique aux graminées dont la rhinite en période de pollinisation du bouleau ne s’accompagnait pas d’une positivité pour rBet v 1 ![]()
Une large réactivité croisée existe entre pollens de graminées et entre allergènes d’un même groupe. Cette réactivité croisée suit à peu près la proximité des pollens sur le plan taxonomique (cf. tableau plus haut).
Il faut tenir compte malgré tout de l’exposition des patients aux pollens environnants, même en cas d’exposition passée : ainsi il a été trouvé 10,8 % de TC positifs pour l’herbe de Bahia (Paspalum notatum) chez des sujets Martiniquais vivant en France métropolitaine, alors que cette graminée ne pousse qu’en climat de type tropical ![]() .
.
La réactivité pour les différents allergènes des graminées est, bien sûr, marquée par une forte individualité. Chez les sujets polysensibilisés (différents pollens, pollens et aliments) la réactivité pour des panallergènes est nettement accrue : c’est le cas pour les allergènes du groupe 7 (polcalcines) et du groupe 12 (profilines).
Peu d’études ont suivi l’évolution des réactivités au cours du temps chez le même patient. Des résultats semblent montrer une relative fixité des réactivités pour des allergènes des groupes 1, 2, 4 et 5 ![]() . Ces profils individuels relèvent probablement de caractéristiques génétiques et sont un des arguments invoqués en faveur d’une immunothérapie restreinte aux seuls allergènes auxquels le patient réagit
. Ces profils individuels relèvent probablement de caractéristiques génétiques et sont un des arguments invoqués en faveur d’une immunothérapie restreinte aux seuls allergènes auxquels le patient réagit ![]() .
.
Les allergènes actuellement décrits dans les pollens de graminées représentent seulement une partie des protéines IgE réactives : des allergènes nouveaux, ne correspondant pas à un des 13 groupes, ont été caractérisés, comme Cyn d 24 qui appartient à une famille de protéines de défense végétale, les PR1.
De plus les méthodes traditionnelles d’obtention des extraits allergéniques imposent aux protéines d’être hydrosolubles. Cette sélection technique fait l’impasse sur certaines protéines non hydrosolubles, comme l’a montré Peltre ![]() . Il est vraisemblable que ces protéines IgE réactives ne sont pas que des figurants lors du contact du patient avec les grains de pollens. Il est possible qu’une partie de l’allergénicité reste donc inexplorée et cela est à mettre en parallèle avec les résultats souvent meilleurs obtenus avec les pricks natifs plutôt qu’avec les extraits.
. Il est vraisemblable que ces protéines IgE réactives ne sont pas que des figurants lors du contact du patient avec les grains de pollens. Il est possible qu’une partie de l’allergénicité reste donc inexplorée et cela est à mettre en parallèle avec les résultats souvent meilleurs obtenus avec les pricks natifs plutôt qu’avec les extraits.
Quels sont les allergènes les plus souvent positifs dans les pollens de graminées ?
Les chiffres de prévalence concernent avant tout les pollens de graminées tempérées et fourragères et, au premier chef, la fléole. Cela est notamment du à la disponibilité d’allergènes recombinants pour cette espèce.
Le tableau ci-après donne les pourcentages de positivité retenus en général pour les allergènes de Pooïdées ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() et pour les allergènes d’autres graminées
et pour les allergènes d’autres graminées ![]()
![]()
![]()
![]()
![]() .
.
| Allergène particulier | Positivité | |
|---|---|---|
| Groupe 1 | 76 à 100 % | |
| Groupe 2 | 40 à 70 % | |
| Groupe 3 | 37-70 | |
| Groupe 4 | 50-88 | |
| Groupe 5 | 50-90 | |
| Groupe 6 | Phl p6 | 35-70 |
| Groupe 7 | Phl p7 | 3-12 |
| Cyn d7 | 35 | |
| Groupe 10 | ? | |
| Groupe 11 | Lol p11 | 65 |
| Phl p11 | 32-53 | |
| Groupe 12 | 13-35 | |
| Cyn d12 | 20-47 | |
| Groupe 13 | 25-75 |
Ainsi qu’il sera vu plus loin, certains groupes sont plus ou moins homologues d’un autre groupe : les prévalences des groupes 2 et 3 sont parallèles et suivent celle du groupe 1 ; il en est de même pour le groupe 6 par rapport au groupe 5.
Des réactivités plus fréquentes pour les profilines (groupe 12) sont vues dans le pourtour méditerranéen comparativement à l’Europe centrale ou septentrionale. Cela est net pour un pollen de climat chaud comme le chiendent digité (Cynodon dactylon).
Les prévalences de réactivité du tableau ci-dessus concernent la moyenne des patients. Dans le cas de polyréactivités (graminées, autres pollens et/ou aliments végétaux) la prévalence peut être beaucoup plus élevée : par exemple 50 à 90 % de positivité s’agissant des profilines ![]()
![]()
![]()
![]() .
.
Inversement, les patients mono-polliniques voient leurs réactivités plus faibles : ainsi en cas de mono-pollinose avec les graminées, il a été relevé 35 % de rPhl p5 positifs au lieu des 80 % habituels ![]() .
.
La mono-sensibilisation aux graminées est relativement rare (13-18 % des polliniques selon Mari ![]()
![]() ).
).
La mono-réactivité à un seul allergène est, elle, très rare. Il n’y a guère que le groupe 1 qui montre quelques cas de réactivité isolée (6 %) ![]()
![]() , les autres groupes montrant des taux le plus souvent < 1 % et qui deviennent nuls en cas de pollinose isolée aux graminées
, les autres groupes montrant des taux le plus souvent < 1 % et qui deviennent nuls en cas de pollinose isolée aux graminées ![]() .
.
Enfin, avec les techniques actuelles, certains groupes d’allergènes de graminées semblent absents dans certains pollens :
- chiendent digité (Cynodon dactylon) : pas de groupe 2
 ni de groupe 5
ni de groupe 5 
 ; pas de groupe 5 dans ce pollen ni dans le pollen de maïs
; pas de groupe 5 dans ce pollen ni dans le pollen de maïs 
- le roseau (Phragmites spp.) : pas de groupe 2 ni de groupe 5



- pour l’herbe de Bahia (Paspalum notatum), certains travaux ont montré la présence d’un groupe 5

 quand d’autres concluaient à une absence du groupe 5
quand d’autres concluaient à une absence du groupe 5  .
.
Dans la mesure où ces différentes graminées proviennent d’espèces prospérant en climat chaud et où les anticorps utilisés avaient été validés avec des patients le plus souvent non exposés à ces pollens (Europe centrale, septentrionale), il est plausible de suspecter un biais méthodologique : les allergènes du groupe 5 existeraient bien dans ces pollens mais seraient difficilement révélables de fait d’une homologie affaiblie par la distance taxonomique avec les pollens de climat tempéré.
Fait important : jusqu’à présent les travaux réalisés n’ont pu déceler de LTP dans les pollens de graminées ![]() .
.
Les allergènes du groupe 1 :
Ce sont des bêta-expansines.
Les expansines sont des protéines intervenant dans l’expansion de la cellule végétale par remodelage du réseau des polysaccharides de la paroi ![]() .
.
Il a été supposé que ces protéines avaient une activité enzymatique papain-like ![]() contribuant à leur allergénicité
contribuant à leur allergénicité ![]()
![]() , à l’image des allergènes du groupe 1 des acariens
, à l’image des allergènes du groupe 1 des acariens ![]() . Mais ces allergènes sont dénués d’une réelle activité enzymatique pour d’autres auteurs
. Mais ces allergènes sont dénués d’une réelle activité enzymatique pour d’autres auteurs ![]()
![]()
![]() .
.
Par contre, une activation des cellules épithéliales alvéolaires, avec libération d’IL-8, IL-6 et TGF-béta, a été suggérée ![]() .
.
Comme pour d’autres groupes, les allergènes du groupe 1 se présentent dans le même pollen, sous de nombreuses isoformes ![]()
![]() . Certaines ont un point isoélectrique acide, d’autres basique. La glycosylation est différente aussi entre les isoformes
. Certaines ont un point isoélectrique acide, d’autres basique. La glycosylation est différente aussi entre les isoformes ![]() .
.
La réactivité croisée entre allergènes du groupe 1 dépend de l’éloignement taxonomique et donc des pourcentages d’identité (ex. 70% Cyn d 1-Lol p 1 ![]() , 66% Pas n 1-Phl p 1
, 66% Pas n 1-Phl p 1 ![]() )
) ![]()
![]() .
.
Malgré tout, en règle générale, Cyn d 1 est positif quand Phl p 1 est positif et, ce, même en l’absence de Cynodon dans l’environnement du patient ![]() .
.
Des recombinants d’allergènes du groupe 1 ont été obtenus dans E.coli, mais ils montrent parfois une réactivité inférieure à l’allergène naturel correspondant ![]()
![]()
![]() .
.
Une réactivité croisée a été notée entre Phl p 1 et Art v 1 (armoise) dont l’explication reste inconnue (chaînes O-glucidiques ?) ![]() .
.
Les allergènes des groupes 2 et 3 :
Les groupes 2 et 3 sont proches, tant en masse (environ 11 kDa) qu’en séquence peptidique. Ils ont aussi une ressemblance avec la portion C-terminale des allergènes du groupe 1 (env. 40 % d’identité) ![]()
![]()
![]() .
.
Il est fréquent que la positivité pour Phl p1 s’accompagne d’une positivité pour Phl p2 et 3 ![]() . Et une réactivité croisée a été montrée
. Et une réactivité croisée a été montrée ![]() .
.
Les pollens de graminées céréalières seraient moins riches en groupe 2 que les graminées fourragères ![]() .
.
Les allergènes du groupe 4 :
Les travaux récents confirment l’hypothèse de Leduc ![]() quant à l’homologie des allergènes du groupe 4 avec des protéines à défense végétale appelées "berberine-bridge enzymes" (BBE)
quant à l’homologie des allergènes du groupe 4 avec des protéines à défense végétale appelées "berberine-bridge enzymes" (BBE) ![]()
![]() . L’homologie reste modeste cependant (35-45 % d’identité).
. L’homologie reste modeste cependant (35-45 % d’identité).
Plusieurs travaux ont tenté de rapprocher les allergènes du groupe 4 d’autres sortes d’allergènes :
- une protéine de 60 kDa de l’armoise (initialement dénommée Art v 1) croise avec des protéines de 30-80 kDa
 ou 40 kDa
ou 40 kDa  dans la fléole.
dans la fléole. - Un anticorps anti-Phl p4 reconnaît des protéines dans de nombreux pollens et dans des aliments comme la pomme, le céleri, etc….

- Phl p4 a 25 à 80 % d’identité avec différents fragments d’Api g 5 (céleri)

- Une réactivité croisée existerait entre Phl p 4 et Amb a 1 (ambroisie) qui n’est pas due qu’à des CCD
 .
.
Ces résultats pourraient déboucher sur la reconnaissance d’un domaine homologue entre le groupe 4 des graminées et des protéines diverses (pollens, aliments), n’appartenant éventuellement pas à la même famille moléculaire.
Les allergènes du groupe 4 se présentent sous différentes isoformes ![]()
![]()
![]() et ont entre eux une forte homologie :
et ont entre eux une forte homologie :
- 95 % d’identité entre allergènes des pollens de céréales,
- 83 % entre Phl p 4 et céréales
- et même 72 % entre Phl p 4 et Cyn d 4 (chiendent digité)


 .
.
Contrairement à d’autres groupes, les allergènes du groupe 4 ont été montrés présents dans toutes les graminées. Ils sont cross-réactifs entre eux.
Les allergènes du groupe 4 sont glycosylés ![]()
![]() et leurs chaînes glucidiques sont une source importante d’IgE-réactivité
et leurs chaînes glucidiques sont une source importante d’IgE-réactivité ![]()
![]() , et donc de réactivité CCD.
, et donc de réactivité CCD.
Par exemple, Mari montre que, parmi des sujets CAP positifs mais TC négatifs pour la fléole, 58 % s’avèrent positifs pour nPhl p 4 s’ils sont CCD+ mais 0 % s’ils sont CCD- ![]() .
.
L’utilisation de l’allergène naturel nPhl p 4 pour le diagnostic in vitro est donc sujette à une interférence par des IgE anti-CCD.
Les allergènes du groupe 5 :
Ces protéines semblent restreintes aux seuls pollens de Pooïdées ![]() . On ne connaît pas bien leur fonction biochimique : Phl p 5.02 aurait une activité topoisomérase (ribonucléase)
. On ne connaît pas bien leur fonction biochimique : Phl p 5.02 aurait une activité topoisomérase (ribonucléase) ![]() . Ils n’ont pas été retrouvés dans certaines graminées comme le Cynodon.
. Ils n’ont pas été retrouvés dans certaines graminées comme le Cynodon.
Les allergènes du groupe 5 se présentent volontiers sous des formes assez différentes, autrefois dénommées "a" et "b".
On connaît ainsi Phl p 5.01 (= Phl p 5a) et Phl p 5.02 (= Phl p 5b), Lol p 5.01 et Lol p 5.02 (avec seulement 67 % d’identité), etc… ![]()
![]() . Les pourcentages d’identité entre isoformes de Pha a 5 (alpiste) vont de 40 à 82% !
. Les pourcentages d’identité entre isoformes de Pha a 5 (alpiste) vont de 40 à 82% ! ![]()
Cette hétérogénéité conduit à des masses assez variables d’un pollen à un autre (28 à 38 kDa) ainsi qu’à des contenus eux aussi variables (de 1 à 4) ![]()
![]() .
.
Les dénominations de ces allergènes ont été le reflet de ces complexités : Lol p 5 était précédemment Lol p 1b ou Lol p 9, Poa p 5 était Poa p 9. Il a aussi été décrit Lol p 5c ![]() .
.
Si, globalement, les allergènes du groupe 5 semblent porteurs d’une forte allergénicité, des différences existent entre les isoformes et, par exemple, un anticorps anti-Phl p 5.02 reconnaît Hol l 5.01 (pollen de houlque) mais pas Hol l 5.02 ![]() .
.
Les allergènes du groupe 5, probablement présents à l’état naturel sous forme de dimères ![]() , montrent une certaine instabilité
, montrent une certaine instabilité ![]() . Il est possible que cela corresponde à la présence indispensable d’un ligand
. Il est possible que cela corresponde à la présence indispensable d’un ligand ![]() .
.
Si cela peut poser problème au niveau des extraits, cette instabilité s’est révélée porteuse d’une allergénicité augmentée dans un contexte particulier : en présence de sécrétions nasales Phl p 5 se fragmente en portions de 10-20 kDa qui, collectivement, donnent des tests cutanés plus importants que Phl p 5 lui-même ![]() .
.
En CAP, le recombinant rPhl p 5 correspond à la séquence de Phl p 5.02 (= Phl p 5b).
Les allergènes du groupe 6 :
En fait, pour l’instant, il ne s’agit que d’un seul allergène, Phl p 6 (fléole).
L’existence de Poa p 6 (pâturin) reste à démontrer bien que cet allergène soit listé dans Allergome.
Phl p 6 a quelque homologie avec une portion C-terminale de Phl p 5 ![]()
![]() .
.
De fait, une réactivité croisée, bien qu’inconstante, est trouvée entre Phl p 6 et Phl p 5 ![]()
![]()
![]() . Il est possible que ces 2 allergènes aient eu un ancêtre commun
. Il est possible que ces 2 allergènes aient eu un ancêtre commun ![]() .
.
On connaît 2 isoformes et 6 variants pour Phl p 6 ![]() .
.
Les allergènes du groupe 7 :
Ce sont des polcalcines, c’est-à-dire des protéines liant le calcium (CaBP = calcium binding proteins).
Bien que classés "2-EF hand" comme les parvalbumines des poissons, car possédant 2 sites de liaison pour le calcium, on n’a pu montrer de réactivité croisée entre les polcalcines des végétaux et les parvalbumines.
Il est à noter que, jusqu’à présent, les polcalcines semblent restreintes aux pollens : contrairement aux profilines on ne connaît pas de polcalcine allergénique dans les aliments d’origine végétale.
Par contre, les polcalcines sont des panallergènes polliniques : on en retrouve dans de très nombreux autres pollens (bouleau, olivier, ambroisie, armoise, etc…).
Cette particularité, associée à la faible prévalence d’IgE-réactivité (d’allergénicité ?) des polcalcines contribue à leur statut de marqueur d’une polysensibilisation pollinique. Pour Mari ![]() Phl p 7 n’est positif que si le patient est sensibilisé à d’autres pollens en plus des graminées.
Phl p 7 n’est positif que si le patient est sensibilisé à d’autres pollens en plus des graminées.
Ainsi, bien que l’homologie avec la polcalcine du bouleau, Bet v 4, ne soit pas très élevée (60-70 %) ![]()
![]() , on note une bonne corrélation in vitro entre Phl p 7 et Bet v 4
, on note une bonne corrélation in vitro entre Phl p 7 et Bet v 4 ![]() .Il semble inutile de tester à la fois ces 2 allergènes : un seul suffit.
.Il semble inutile de tester à la fois ces 2 allergènes : un seul suffit.
Certains allergènes du groupe 7 se distinguent aussi par une conformation spatiale particulière (dimère tête-bêche ![]() ) dépendante de la présence ou non de calcium
) dépendante de la présence ou non de calcium ![]() .
.
En l’absence de calcium l’IgE-réactivité a été montrée fortement réduite in vitro ![]()
![]()
![]() Est-ce que cela a une influence aussi dans le cas des extraits pour TC ?
Est-ce que cela a une influence aussi dans le cas des extraits pour TC ?
Les allergènes du groupe 10 :
Des travaux anciens ont montré la présence de protéines IgE-réactives de 12 kDa ayant une activité cytochrome C ![]() . Ces allergènes ont été dénommés Lol p 10 (ivraie) et Poa p 10 (pâturin).
. Ces allergènes ont été dénommés Lol p 10 (ivraie) et Poa p 10 (pâturin).
Par la suite, des études permettant de mieux évaluer ces protéines n’ont pas vu le jour, de sorte que la place de ces protéines dans l’allergénicité des pollens de graminées reste très vague.
Une réactivité croisée avec les cytochromes C fungiques (ex. Cur l 3 de Curvularia) a été avancée ![]() , mais non démontrée.
, mais non démontrée.
Les allergènes du groupe 11 :
Ces glycoprotéines ont été données comme appartenant à une famille d’inhibiteurs trypsiques ![]() , mais cela est contesté
, mais cela est contesté ![]() .On a décrit Phl p 11 (fléole) et Lol p 11 (ivraie) : ces 2 allergènes ont 95 % d’identité. Par contre l’homologie avec l’inhibiteur de Kunitz présent dans le soja n’est que de 32 %.
.On a décrit Phl p 11 (fléole) et Lol p 11 (ivraie) : ces 2 allergènes ont 95 % d’identité. Par contre l’homologie avec l’inhibiteur de Kunitz présent dans le soja n’est que de 32 %.
On rattache souvent les allergènes du groupe 11 des graminées à ceux du groupe 1 des Oléacées, les Ole e 1-like. Cependant l’homologie est là aussi très limitée (env. 34-40 %) et Phl p 11 ne montre pas de réactivité croisée avec Ole e 1 ![]() .
.
Pour Mari, les allergènes du groupe 11 ne sont pas des panallergènes ![]() .
.
Des protéines ressemblant à Phl p 11 se trouvent dans d’autres pollens de graminées (maïs, riz, alpiste), avec une homologie assez bonne entre elles (69-79 %) mais plus limitée avec Phl p 11 (45-49 %).
L’allergénicité des protéines du groupe 11 est très mal connue.
Les allergènes du groupe 12 :
Ce sont des profilines.
Comme les allergènes du groupe 7, ceux du groupe 12 sont considérés comme un bon marqueur d’une polysensibilisation ![]() . Là aussi, une corrélation est trouvée entre la réactivité pour Phl p 12 et celle pour la profiline du bouleau, Bet v 2, chez les mêmes patients
. Là aussi, une corrélation est trouvée entre la réactivité pour Phl p 12 et celle pour la profiline du bouleau, Bet v 2, chez les mêmes patients ![]() .
.
Van Ree estime que Lol p 12 (ivraie) a une meilleure sensibilité que Bet v 2 ![]() . Ce qui a été contesté
. Ce qui a été contesté ![]() . Il est probable que la profiline la plus adaptée à tester soit celle du pollen dominant dans l’environnement du patient.
. Il est probable que la profiline la plus adaptée à tester soit celle du pollen dominant dans l’environnement du patient.
Du fait de la présence de profilines dans les pollens et dans les aliments végétaux, la positivité pour une profiline pollinique prendra une valeur particulière chez un patient présentant une allergie à des fruits et légumes et, par ailleurs, négatif pour le bouleau, le latex et les LTP ![]() .
.
Les allergènes du groupe 13 :
Ces allergènes ont été confondus avec ceux du groupe 4 car migrant au même endroit en immunoblot. Ces allergènes sont pourtant distincts ![]() et ne croisent pas avec ceux du groupe 4
et ne croisent pas avec ceux du groupe 4 ![]() .
.
Ce sont des polygalacturonases. On en a caractérisé dans toutes les espèces de graminées, les pourcentages d’identité chutant cependant avec l’éloignement taxonomique : 68 % pour Zea m 13 (maïs) et 58 % pour Ory s 13 (riz) comparativement à Phl p 13 (fléole). Une réactivité croisée est cependant possible entre Zea m 13 et Phl p 13 ![]() .
.
Des polygalacturonases (PG) sont connues dans des pollens autres que les graminées et dans de nombreux aliments végétaux. Mais les pourcentages d’identité entre exo-PG (pollens de graminées) et endo-PG (aliments) sont faibles (36-41 %), de même qu’entre les exo-PG des graminées et les exo-PG des dicotylédones. C’est la raison pour laquelle le groupe 13 des graminées a été suggéré comme un marqueur spécifique d’une sensibilisation aux graminées ![]() .
.
Mais cette utilisation semble difficile en pratique car les PG des pollens de graminées sont glycosylées ![]()
![]() et donc susceptibles d’être positivées par la simple présence d’IgE anti-CCD.
et donc susceptibles d’être positivées par la simple présence d’IgE anti-CCD.
Autres allergènes :
– Une glycoprotéine de 21 kDa, Cyn d 24, a été montrée IgE-réactive dans le pollen de chiendent digité (Cynodon dactylon).
- C’est une protéine PR-1 de défense végétale. On connaît des protéines de ce type dans d’autres végétaux, dont des céréales (45-50 % d’identité)
 . Pour le moment seuls Cyn d 24 et Cuc m 3 (melon) ont une IgE-réactivité prouvée parmi les protéines PR-1.
. Pour le moment seuls Cyn d 24 et Cuc m 3 (melon) ont une IgE-réactivité prouvée parmi les protéines PR-1. - La prévalence de positivité pour Cyn d 24 a été donnée pour 65 % parmi les patients positifs pour le chiendent digité
 . Mais des travaux complémentaires sont nécessaires pour préciser l’importance de Cyn d 24.
. Mais des travaux complémentaires sont nécessaires pour préciser l’importance de Cyn d 24.
– D’autres protéines IgE réactives (et glycosylées) ont été repérées dans le pollen de chiendent digité :
- une protéine de 46 kDa qui pourrait posséder une activité de cytochrome C oxydase

- une 66 kDa qui a une homologie avec une ascorbate oxydase
 .
.
– Dans le pollen de maïs un homologue d’Amb a 1 (ambroisie) et de LAT59 (pollen de tomate) a été suspecté ![]() . Ce serait une pectate lyase.
. Ce serait une pectate lyase.
Quels sont les allergènes les plus importants cliniquement ?
Il est classique de donner les allergènes des groupes 1 et 5 comme les plus importants car :
- les plus fréquemment positifs
- et/ou représentant la majeure part de l’IgE-réactivité constatée en CAP pour le pollen lui-même
 .
.
Cependant, ces tests in vitro ne sont pas garants d’un pouvoir équivalent in vivo.
Ainsi, une étude récente ![]() a montré :
a montré :
- qu’il n’y avait pas corrélation entre la réactivité des allergènes de fléole in vitro et leur pouvoir réactogène en TC
- et que le pouvoir réactogène cutané de rPhl p 2 était, en fait, supérieur à ceux de rPhl p 1 et de rPhl p 5. Rapporté à rPhl p 2, le pouvoir réactogène médian de rPhl p 5 était de 77% et celui de rPhl p 1 de 40%.
- par ailleurs, les mêmes ratios observés pour rPhl p 4 (15%) et rPhl p 13 (12%) montraient que ces deux allergènes contribuaient peu à la réponse clinique.
Réactivité croisée entre pollens de graminées
Une large réactivité croisée entre pollens de graminées est fortement suggérée par la fréquence des multi-positivités à différentes espèces de graminées. Cela se traduit aussi dans le parallélisme des résultats in vitro, pour autant que les espèces soient proches taxonomiquement.
Ainsi, une étude ayant porté sur plus de 5000 sérums a montré une corrélation très significative entre le résultat pour la fléole et celui pour 9 autres Pooïdées (r = 0,89 à 0,97). Ces corrélations étaient nettement plus lâches entre fléole et Cynodon (r = 0,43) ou roseau commun (Phragmites, r = 0,46), ces deux pollens présentant une réactivité plus basse que celle pour la fléole le plus souvent.
Les études de réactivité croisée proprement dites sont souvent difficiles à rapprocher les unes des autres : mode de sélection des patients, nombre de patients souvent modestes, pollens environnants très dépendants de la géographie, méthodes d’inhibition parfois qualitatives, parfois quantitatives.
Sans que cela se traduise obligatoirement sur le plan clinique, les doses nécessaires d’un pollen pour inhiber l’IgE-réactivité vis à vis d’un autre pollen seraient aussi à prendre en compte : par exemple la dose du pollen A permettant une inhibition d’au moins 50 % (IC50) du pollen B est parfois sans commune mesure avec la dose homologue, c’est-à-dire celle de B pour s’inhiber soi-même. Des facteurs de 100 ou plus, sont possibles rendant l’interprétation de la réaction croisée délicate sur le plan clinique si le patient est en contact avec des doses peu différentes de A et de B.
Ces écarts immunologiques de réactivité croisée ont été relevés par exemple entre la fléole et d’autres graminées fourragères ![]() ou entre pollen de maïs et pollens d’autres céréales
ou entre pollen de maïs et pollens d’autres céréales ![]() .
.
Une vue d’ensemble peut cependant être déduite des travaux publiés :
- en général, les graminées fourragères (ivraie, dactyle, pâturin, etc…) ont le dessus sur les autres graminées, qu’elles soient exotiques ou céréalières. En ce sens, les graminées fourragères inhiberont d’autres pollens qui, de leur côté, ne parviendront pas toujours voire jamais à produire l’inhibition inverse.
- Cette dissymétrie peut provenir d’une allergénicité plus prononcée de certains pollens, ou de la nature des pollens majoritairement présents dans l’environnement des patients.
- Mais le plus souvent elle indique le(s) pollen(s) qui a (ont) été à l’origine de la sensibilisation, c’est-à-dire les pollens majoritairement présents dans l’environnement du patient : ce sont ceux-là pour lesquels les IgE du patient sont les plus adaptées et qui inhibent plus facilement les autres pollens.
- les graminées fourragères et les graminées céréalières (Triticées et avoine) montrent des réactivités croisées aisées.
- Les céréalières semblent cependant dépendre d’une sensibilisation aux fourragères et tester le pollen de blé, de seigle, etc… en plus du dactyle, de l’ivraie, etc… n’est pas utile dans la majorité des cas.
- Le maïs est un peu à part du fait de son éloignement taxonomique : en général, il inhibe peu les autres pollens de graminées tempérées.
- La réactivité croisée du pollen de riz avec d’autres graminées reste grandement inconnue.
- parmi les graminées adaptées à des climats chauds, il faut citer le cas du chiendent digité (Cynodon dactylon).
- la réactivité croisée entre graminées tempérées et les autres graminées tropicales a surtout été étudiée ailleurs qu’en Europe.
- En général les graminées Pooïdées inhibent les graminées tropicales, lesquelles proviennent d’une branche séparée parmi les Poacées (le clade PACC).
- Les études de réactivité croisée entre différentes graminées tropicales ont donné des résultats disparates, pas toujours parallèles à la classification taxonomique.
Réactivités croisée entre pollens de graminées et d’autres pollens
Parmi les groupes d’allergènes de graminées, certains n’ont été identifiés que dans les graminées et ne peuvent être à l’origine de réactions croisées avec d’autres pollens (à moins d’être porteurs de CCD) : groupes 1, 2, 3, 5 et 6.
Un doute subsiste au sujet de la présence d’homologues du groupe 4 des graminées dans d’autres pollens.
Les allergènes des groupes 11 et 13 ont bien des homologues dans les pollens d’Oléacées, de Cupressacées ou de diverses herbacées mais leur réactivité croisée avec ces homologues n’a pu être démontrée (les pourcentages d’identité entre protéines sont faibles).
L’essentiel de la réactivité croisée entre pollens de graminées et autres pollens a pour origine les polcalcines (groupe 7) et les profilines (groupe 12). Il faut y ajouter les CCD dans le cas des tests in vitro.
Les polcalcines et les profilines sont des panallergènes : on a identifié ces allergènes dans pratiquement tous les pollens allergisants, à l’exception notoire des Cupressacées (pour le moment).
Un test cutané peut être positivé par réactivité croisée : par exemple, le bouleau se voir positivé par une sensibilisation à des polcalcines de graminées et d’herbacées. Cela a été montré pour des pollens absents de l’environnement du patient, comme le bouleau à Madrid ou l’ambroisie à Rome ![]() .
.
En pratique, des tests cutanés positifs concordant mal avec la clinique et/ou l’environnement du patient peuvent provenir :
- d’une sensibilisation ancienne, le patient ayant été au contact précédemment avec ces pollens
- d’une réactivité croisée due à des panallergènes. Cela pourra être vérifié en testant in vitro l’IgE-réactivité vis-à-vis de rPhl p 7 (polcalcine) et de rPhl p 12 (profiline). Il est à noter que la sensibilisation à ces allergènes se voit le plus souvent chez un patient poly-réactif (différents pollens, ou pollens et aliments végétaux).
Réactivités croisées entre pollens de graminées et aliments
La question est souvent posée en ce qui concerne les farines de céréales du fait de la proximité taxonomique, voire de l’identité d’espèce entre pollen et aliment. Ce point est traité ailleurs.
Pour d’autres aliments, il est possible que les graminées jouent un rôle dans la genèse d’une allergie alimentaire, en règle générale limitée à un syndrome oral. C’est avant tout la responsabilité de profilines. Et donc plus fréquent dans les environnements de type méditerranéen où la prévalence d’une réactivité aux profilines est importante.
Par exemple, l’étude épidémiologique EXPO menée en Espagne ![]() a montré que si 38% des patients rapportant un syndrome oral étaient positifs pour rMal d 4 (profiline de pomme), la prévalence de réactivité pour les graminées chez ces patients était nettement supérieure (74% pour Phl p 1, 56% pour Phl p 5) à celle pour les LTP (33% pour rPru p 3).
a montré que si 38% des patients rapportant un syndrome oral étaient positifs pour rMal d 4 (profiline de pomme), la prévalence de réactivité pour les graminées chez ces patients était nettement supérieure (74% pour Phl p 1, 56% pour Phl p 5) à celle pour les LTP (33% pour rPru p 3).
Le diagnostic d’une sensibilisation aux graminées
Ce diagnostic ne pose pas de problème en général : saisonnalité et caractéristiques des symptômes, tests cutanés.
Les techniques in vitro peuvent être affectées par l’interférence d’IgE anti-CCD (cf. Graminées et CCD). Dans l’hypothèse où des tests in vitro sont jugés utiles, mieux vaut utiliser des allergènes recombinants (non glycosylés) que des extraits globaux.
Bien que la fléole ne soit pas l’exacte réplique de tous les pollens de Pooïdées, la mesure de l’IgE-réactivité pour rPhl p 1 et/ou rPhl p 5 est utile pour confirmer une sensibilisation, comme l’a montré Valenta dès 1992 ![]() .
.
Mais ces 2 allergènes recombinants peuvent s’avérer insuffisants pour remplacer les extraits de graminées tropicales (ou de tendance tropicale comme le chiendent digité Cynodon dactylon) soit parce que la réactivité croisée avec ces pollens est insuffisante, soit parce que ces pollens sont déficients en certains allergènes, tel le groupe 5.
La sensibilité d’une mesure associant Phl p 1 et Phl p 5 est de 87 % comparativement aux tests cutanés. Ajouter d’autres recombinants en systématique apporte peu : 93 % de sensibilité en associant 8 recombinants différents de fléole ![]() . Mais, ponctuellement, si rPhl p 1 et rPhl p 5 sont négatifs, on peut tenter de compléter l’exploration avec rPhl p 2 et/ou nPhl p 4, en sachant que des IgE anti-CCD peuvent perturber le résultat de l’allergène naturel nPhl p 4.
. Mais, ponctuellement, si rPhl p 1 et rPhl p 5 sont négatifs, on peut tenter de compléter l’exploration avec rPhl p 2 et/ou nPhl p 4, en sachant que des IgE anti-CCD peuvent perturber le résultat de l’allergène naturel nPhl p 4.
Un allergène recombinant hybride, associant les séquences des groupes 1, 2, 5 et 6 de fléole, a été testé avec succès ![]() : sa sensibilité était de 97%
: sa sensibilité était de 97%
Si l’on sait que la corrélation entre le résultat chiffré des mesures in vitro (les "kU/l") et la réalité clinique est mauvaise ![]() , il faut rappeler aussi que les contenus en allergènes varient de façon considérable entre les extraits commercialisés pour tests cutanés.
, il faut rappeler aussi que les contenus en allergènes varient de façon considérable entre les extraits commercialisés pour tests cutanés.
Et cela se traduit par des variations de réponses en TC, chez le même patient, d’un extrait industriel à un autre, comme le montre le tableau ci-dessous ![]() :
:
Ratio = rapport entre la surface de la papule avec l’extrait sur celle avec l’histamine
| Patient | Ratio le plus faible | avec extrait n° | Ratio le plus élevé | avec extrait n° |
|---|---|---|---|---|
| a | 0,45 | 1 | 1,03 | 3 |
| b | 0,61 | 2 | 2,91 | 1 |
| c | 0,90 | 3 | 2,54 | 4 |
| d | 0,33 | 1 | 2,33 | 2 |
| e | 0,88 | 4 | 3,06 | 1 |
| f | 5,06 | 2 | 11,2 | 4 |
| g | 1,06 | 3 | 1,62 | 4 |
| h | 0,87 | 3 | 2,6 | 4 |
| i | 1,87 | 3 | 2,6 | 1 |
| j | 4,28 | 3 | 8,76 | 1 |
Les tests in vitro ont donc leur place, particulièrement au moment de décider d’une immunothérapie.
Pollens de graminées et CCD
Les pollens de graminées sont probablement le vecteur le plus courant d’épitopes glucidiques. En effet, les graminées poussent sous tous les climats et plusieurs de leurs allergènes montrent une IgE-réactivité glucidique : groupes 1, 4, 11 et 13 notamment.
L’interférence des épitopes glucidiques dans les résultats in vitro ne se limite pas aux seuls allergènes : d’autres glycoprotéines dénuées d’allergénicité mais présentes dans l’extrait testé peuvent, elles aussi, être la cible des IgE anti-glycannes du patient ![]() .
.
La pollinose aux graminées s’accompagne donc souvent d’une réactivité de type CCD. La prévalence de cette réactivité est estimée entre 15 et 30 % ![]()
![]()
![]()
![]() , sachant qu’il est difficile de séparer la part prise par d’autres pollens chez des patients qui sont dans l’ensemble assez rarement mono-polliniques aux graminées.
, sachant qu’il est difficile de séparer la part prise par d’autres pollens chez des patients qui sont dans l’ensemble assez rarement mono-polliniques aux graminées.
De fait, la prévalence d’une "positivité CCD" augmente fortement parmi les patients poly-polliniques :
- 63 % de tests broméline positifs chez des sujets sélectionnés sur la positivité pour nPhl p 4
 .
. - 56 % de tests dactyle encore positifs après coupure des protéines par la protéinase K

- 10,4 fois plus de faux positifs fléole (= avec TC négatifs) chez les sujets "CCD positifs"

- une bien meilleure corrélation entre rPhl p 12 (ou rBet v 2) et la broméline qu’entre rPhl p 1 (ou 5) et la broméline
 montrant le lien entre poly-pollinose, sensibilisation à des panallergènes (ici profilines) et génération d’IgE anti-CCD.
montrant le lien entre poly-pollinose, sensibilisation à des panallergènes (ici profilines) et génération d’IgE anti-CCD.
La broméline est souvent utilisée comme glyco-reporter : cette protéine est relativement bien adaptée aux allergènes des graminées car, provenant d’une plante monocotylédone également, l’ananas.
- Elle porte une chaîne glucidique de type MUXF largement répandue dans les allergènes de graminées (NB : pour la signification des abréviations comme "MUXF" données aux chaînes glucidiques, se reporter à l’article sur les CCD).
- En plus des chaînes de type MUXF on trouve des MMXF mais pas de chaînes pluri-mannosylées dans les pollens de graminées


 .
.
On a aussi montré la présence d’arabino-galactannes dans ces pollens.
- On sait que l’allergène Art v 1 de l’armoise a un domaine C-terminal comportant des chaînes glucidiques formées d’arabinose et de galactose et que ces structures glucidiques sont reconnues par les IgE des patients polliniques à l’armoise

- Mais dans le cas des pollens de graminées une IgE-réactivité des arabino-galactannes n’a pu être décelée pou le moment
 .
.
Si les pollens, et notamment ceux des graminées, sont capables de susciter la génération d’IgE anti-glycannes, la positivité pour un glyco-reporter comme la broméline peut avoir d’autres causes.
- Ainsi, Kochuyt
 montre que l’allergie aux venins d’hyménoptères peut, à elle seule, causer des faux positifs in vitro pour de nombreux pollens, dont les graminées : environ 40 % chez des patients allergiques à l’abeille et à la guêpe !
montre que l’allergie aux venins d’hyménoptères peut, à elle seule, causer des faux positifs in vitro pour de nombreux pollens, dont les graminées : environ 40 % chez des patients allergiques à l’abeille et à la guêpe !
La relation entre CCD et graminées est donc double :
- il faut tenir compte d’une pollinose dans l’interprétation d’autres tests in vitro (aliments végétaux, latex, hyménoptères)
 ;
; - mais il faut aussi garder à l’esprit la possibilité de faux positifs in vitro chez des patients allergiques aux hyménoptères.
Graminées et immuno-thérapie
Désensibiliser ou non ?
Valenta a proposé de moduler l’indication d’une désensibilisation en fonction de la positivité du patient vis-à-vis des profilines et/ou des polcalcines. Trois niveaux sont ainsi définis ![]() :
:
- bonne indication : positivité pour rPhl p 1 et/ou rPhl p 5 et négativité pour rPhl p 12 (profiline) et rPhl p 7 (polcalcine)
- faible indication : rPhl p 1 et/ou rPhl p 5 sont positifs mais rPhl p 12 et/ou rPhl p 7 sont positifs aussi
- non indication : rPhl p 1 et rPhl p 5 sont tous deux négatifs
Plus récemment, Valenta a assoupli sa position dans le cadre d’une réactivité aux graminées en zone méditerranéenne ![]() . L’immuno-thérapie est, cette fois, indiquée avec rPhl p 1 et/ou rPhl p 5 positifs même si rPhl p 12 et/ou rPhl p 7 sont positifs.
. L’immuno-thérapie est, cette fois, indiquée avec rPhl p 1 et/ou rPhl p 5 positifs même si rPhl p 12 et/ou rPhl p 7 sont positifs.
Il est vrai que la fréquence de positivité pour les profilines dans le sud de l’Europe rendait la règle initiale difficile à soutenir …
De plus, les polliniques aux graminées avec TC positif en profiline ont été montrés avoir souvent une atteinte clinique plus prononcée ![]() .
.
Désensibiliser avec quel(s) pollen(s) ?
Il semble naturel de désensibiliser avec les pollens auxquels le patient est exposé. Le choix du (ou des) pollen(s) sera donc différent en Europe et en Australie ou dans le sud des USA.
Qu’en est-il du Cynodon ?
L’étude EXPO a montré qu’il était très rare d’observer un test Cyn d 1 positif sans positivité pour Phl p 1 ![]() . Et que cela se produisait essentiellement sur la frange méditerranéenne de l’Espagne.
. Et que cela se produisait essentiellement sur la frange méditerranéenne de l’Espagne.
Par ailleurs, une positivité en CAP pour nCyn d 1 n’indique pas obligatoirement une sensibilisation au Cynodon mais plutôt une réactivité croisée avec le groupe 1 des Pooïdées, voire la présence d’IgE anti-CCD ![]() .
.
Si le Cynodon est parfois utilisé en immunothérapie, cela concerne avant tout les régions du monde où ce pollen est fortement présent dans l’environnement du patient. En Europe tempérée, il n’est pas estimé nécessaire d’adjoindre Cynodon aux graminées Pooïdées ![]() .
.
Qu’en est-il justement pour les pollens de Pooïdées ? Est-il préférable d’utiliser un mélange de pollens ? Ou bien un seul d’entre eux suffit pour désensibiliser le patient à l’ensemble des Pooïdées ?
Le débat à ce sujet est en cours. D’emblée on peut noter :
- que tous les patients sont exposés à une large variété d’espèces de graminées … dont une partie seulement a été étudiée, d’ailleurs
- que la répartition des espèces de Pooïdées n’est pas uniforme d’un pays à un autre ou d’une région à une autre. La fléole n’est pas prévalente au point de dominer toutes les autres espèces. Mais il se trouve que le pollen de fléole est le mieux étudié en ce qui concerne ses allergènes et que l’on possède un panel de recombinants de fléole pour le diagnostic.
La logique voudrait que chaque patient reçoive un extrait représentatif des pollens auxquels il est exposé. Ceci n’est pas possible. Aussi, l’habitude a prévalu d’utiliser des mélanges standards de quelques pollens. Ces mélanges sont également mis en œuvre pour les tests cutanés. Par exemple, dans l’étude EXPO dont les résultats viennent d’être présentés et qui a recensé les réactivités polliniques sur le territoire espagnol, un mélange ALK de 5 graminées a été utilisé ![]() .
.
L’habitude, voire l’expérience de l’efficacité, suffisent-elles à justifier l’emploi d’un mélange de pollens plutôt qu’un seul, comme la fléole ?
Les arguments des uns et des autres sont souvent basés sur l’existence de multiples réactivités croisées entre la fléole et les autres Pooïdées ![]()
![]() , ou sur des contenus disparates en allergènes d’un pollen à un autre
, ou sur des contenus disparates en allergènes d’un pollen à un autre ![]()
![]() .
.
Mais la bonne question est de savoir s’il existe ou non une complète réactivité croisée au niveau des cellules T entre pollens de Pooïdées.
La réponse est souvent biaisée par l’emploi de clones T sélectionnés à l’aide d’allergènes de fléole : clones Phl p 1- ou Phl p 5-spécifiques.
Malgré tout, une superposition complète de la fléole avec les autres Pooïdées est improbable. En effet :
- sur 38 clones Phl p 5-spécifiques, 14 étaient activés par Poa p 5 mais pas par Lol p 5 ; pour 2 autres c’était l’inverse ; et pour 2 autres : pas d’activation ni par Poa p 5 ni par Lol p 5

- des résultats similaires ont été observés dans une autre étude
 où 30% des clones Phl p 5-spécifiques n’étaient pas stimulés par Lol p 5 ou Poa p 5 ou Dac g 5
où 30% des clones Phl p 5-spécifiques n’étaient pas stimulés par Lol p 5 ou Poa p 5 ou Dac g 5 - de même pour Phl p 1 : seulement 8 des 19 clones Phl p 1-spécifiques étaient activés par les 4 autres pollens testés
 .
.
Des épitopes T spécifiques d’espèce sont suggérés entre allergènes du groupe 1 ![]() . Et une variabilité des réponses T est vue d’un patient à un autre entre épitopes T (et aussi entre isoformes) de Phl p 5
. Et une variabilité des réponses T est vue d’un patient à un autre entre épitopes T (et aussi entre isoformes) de Phl p 5 ![]() .
.
Si l’on peut montrer qu’effectivement des clones T spécifiques de Phl p 1 ou Phl p 5 sont stimulables par d’autres pollens, ceci est à des degrés divers d’un patient à un autre ![]() .
.
Et quand des résultats chiffrés sont présentés patient par patient on peut constater que la stimulation maximale est parfois supérieure avec un allergène autre que celui de fléole : rapportée à celle suscitée par Phl p 5 chez le même patient, cette stimulation allait de 0,51 à 2,18 avec Poa p 5 et de 0,25 à 2,15 pour Lol p 5 ![]() .
.
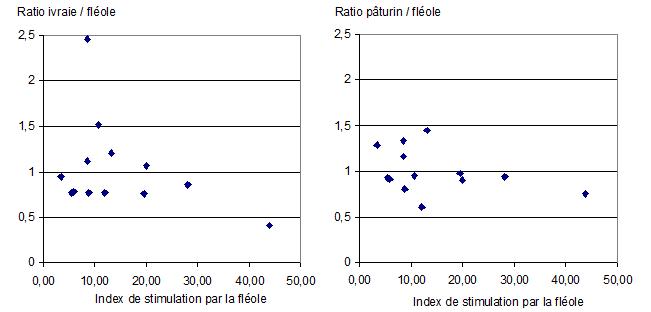
Plus intéressants sont les résultats obtenus dans la même étude avec des extraits et non plus un allergène particulier. Car ce sont bien des extraits qui sont (encore pour le moment) utilisés pour désensibiliser. La figure ci-dessous traduit le ratio de stimulation pollen x/fléole en fonction du degré d’activation constaté pour la fléole ![]() . On peut y constater que, pour certains patients, le ratio est > 1, c’est-à-dire favorable à un autre pollen que la fléole.
. On peut y constater que, pour certains patients, le ratio est > 1, c’est-à-dire favorable à un autre pollen que la fléole.