Accueil / Information / Aller plus loin... / Familles moléculaires / Les polcalcines
Les polcalcines
mercredi 18 août 2010, par
Les polcalcines sont des panallergènes polliniques. Ces petites protéines de 6-10 kDa, non glycosylées, font partie d’une famille dénommé 2EF-hand calcium-binding proteins (2EF-CaBP) : elles possèdent, en effet, 2 sites de liaison pour l’ion calcium.
Il existe de nombreuses sortes de protéines liant le calcium, certaines transportant/tamponnant le calcium, d’autres jouant un rôle dans la régulation cellulaire. Les polcalcines s’inscrivent dans une super-famille d’EF-hand, au sein de laquelle on trouve également ![]()
![]()
![]() :
:
- les parvalbumines (poissons, batraciens)
- les troponines : ex. Tyr p 24 (acarien Tyrophagus), Bla g 6 et Per a 6 (blattes)
- les protéines S100 : ex. Bos d 3 (bœuf)
- les sarcoplasmic calcium-binding proteins (SCBP) : dans les Crustacés
- des allergènes polliniques un peu différents des polcalcines, bien que parfois dénommées aussi polcalcines
 :
:
- des 3EF-hand, ayant donc 3 sites pour lier le calcium : bouleau (Bet v 3), ambroisie (Amb a 10), armoise
- des 4EF-hand : olivier (Ole e 8) et Cupressacées (Cup a 4, Jun o 4, Cry j 4, ..)
- sans être des duplicats des 2EF, les 4EF sont plus apparentées aux polcalcines 2EF que ne le sont les 3EF (cf. cladogramme dans
 ).
).
Les polcalcines se différencient d’une autre catégorie de panallergènes des plantes, les profilines : jusqu’à présent on n’a pas identifié de polcalcines IgE-réactives dans d’autres organes végétaux que les pollens.
Mais, comme les profilines, les polcalcines ont une large capacité de réactivité croisée entre elles. De sorte qu’elles constituent bien une catégorie de panallergènes polliniques et que la sensibilisation d’un patient à la polcalcine du pollen X risque fort de générer une réactivité TC, in vitro) vis-à-vis des pollens Y, Z, etc.., même si le patient n’est pas exposé à ces pollens.
Ce qui, bien entendu, va embrouiller le diagnostic, les résultats des tests étant alors volontiers en désaccord avec les données cliniques (ex. période(s) de l’année gênant le patient) ![]() .
.
Le tableau ci-dessous montre les % d’identité séquentielle entre polcalcines : ces taux élevés d’homologie sont confirmés au niveau tridimensionnel (épitopes conformationnels) ![]() et justifient la large réactivité croisée observée entre ces allergènes
et justifient la large réactivité croisée observée entre ces allergènes ![]()
![]()
![]()
![]()
![]()
![]() etc ..
etc ..
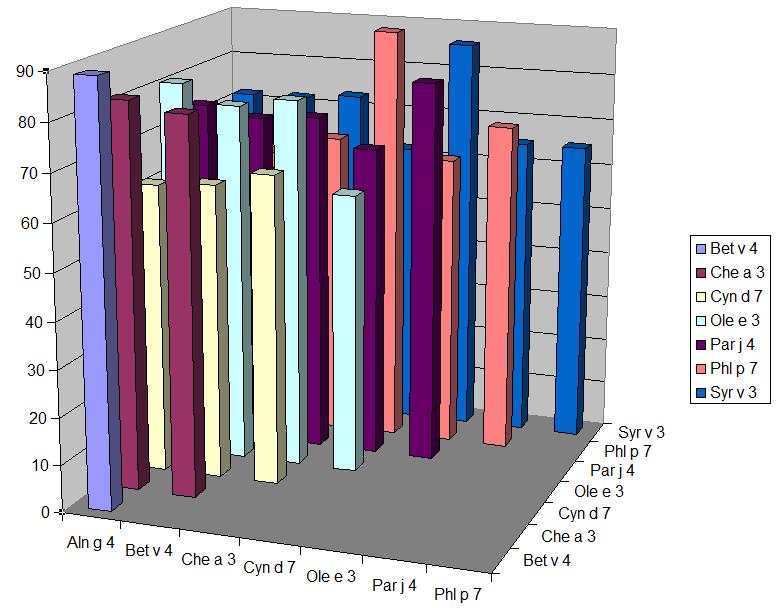
La réactivité croisée entre polcalcines est-elle qu’il est en pratique suffisant de n’en tester qu’une seule. Par exemple une corrélation élevée (r=0,95) a été trouvée entre les résultants chiffrés (kU/l) de rBet v 4 (bouleau) et de rPhl p 7 (fléole) ![]() .
.
Pour le moment sont testables en CAP rBet v 4 et rPhl p 7. Ces tests sont pertinents pour rechercher une réactivité due à des polcalcines dans la plupart des autres pollens : fagales, graminées, Oléacées, armoise, ambroisie, pariétaire, chénopode, ..
Une polcalcine existe dans le pollen de plantain ![]() , mais son IgE-réactivité n’a pas été étudiée. La présence de polcalcines dans les pollens de Cupressacées n’est pas encore établie (NB : ces pollens ont, par contre, des allergènes 4EF-hand).
, mais son IgE-réactivité n’a pas été étudiée. La présence de polcalcines dans les pollens de Cupressacées n’est pas encore établie (NB : ces pollens ont, par contre, des allergènes 4EF-hand).
Les polcalcines (2EF) croisent-elles avec les protéines 3EF ou 4EF ? A priori pas ou peu. Les homologies sont faibles : par exemple les 4 EF comme Cup a 4 (cyprès) ou Ole e 8 (olivier) ont des % d’identité de 48-53% (sur leur portion N-terminale) et 33-34% (sur leur portion C-terminale) avec Phl p 7. Et, bien que peu explorées, les réactivités croisées sont inexistantes ![]()
![]() , ou dans le sens polcalcine -> 3EF ou 4EF et pas l’autre sens
, ou dans le sens polcalcine -> 3EF ou 4EF et pas l’autre sens ![]()
![]() .
.
Une réactivité vis-à-vis des polcalcines est-elle fréquente ? Le tableau ci-dessous rassemble quelques observations de positivités in vitro :
Comme pour les profilines, les prévalences d’IgE-réactivité pour des polcalcines tendent à croître du Nord au Sud de l’Europe. Avec de fortes variations parfois d’une étude à une autre dans un même pays, ce qui est probablement du à des recrutements de patients assez différents. En effet, la réactivité vis-à-vis des polcalcines semble en grande partie corrélée avec une pluri-réactivité pollinique : une cohorte de patients multi-réactifs pour différents pollens rassemblera une proportion accrue de sujets positifs pour les polcalcines.
Ainsi il n’a été observé aucun cas de positivité pour rBet v 4 ou rPhl p 7 parmi des patients mono-réactifs à une seule catégorie de pollens (fagales ou graminées) ![]()
![]()
![]() .
.
A l’inverse, la positivité (TC ou in vitro) pour 5 pollens ou plus s’accompagnait d’une réactivité possible pour les polcalcines ![]() . La figure ci-dessous, tirée d’un autre travail
. La figure ci-dessous, tirée d’un autre travail ![]() , présente la répartition des TC cutanés positifs pour la polcalcine (polc) et la profiline (prof) de palmier chez des patients testés avec 9 pollens différents (arbres, graminées, herbacées) :
, présente la répartition des TC cutanés positifs pour la polcalcine (polc) et la profiline (prof) de palmier chez des patients testés avec 9 pollens différents (arbres, graminées, herbacées) :

On pourra noter que les profilines sont un peu plus « rapidement » positives que les polcalcines à mesure que le nombre de pollens positifs augmente.
Il faut souligner cependant qu’une dissociation entre profilines et polcalcine semble fréquente :
- sur 21 patients positifs in vitro pour une polcalcine et une profiline, seulement 3 étaient simultanément positifs pour les 2 allergènes

- parmi 16 patients présentant un TC positif pour une polcalcine, 10 étaient négatifs en profiline

So l’on ajoute à cela qu’il est très rare d’observer une mono-positivité pour une polcalcine (ex. rBet v 4 positif avec rBet v 1 et rBet v 2 négatifs), on pourra en déduire la pratique suivante :
- en cas de pollens positifs peu concordants avec la clinique, on peut tirer parti de la dissociation possible profiline/polcalcine en testant séparément ces 2 allergènes (ex. rPhl p 12 et rPhl p 7 plutôt que le test combiné rPhl p 12+7). On aura ainsi, au besoin, une information supplémentaire en cas de réactions alimentaires associées (rôle d’une profiline ?)
- il n’est pas pertinent de tester la réactivité aux polcalcines devant une positivité pour 1 ou 2 pollens : cette recherche a toutes chances d’être négative
- de plus, on ignore la part éventuelle qu’une IgE-réactivité pour une polcalcine pourrait prendre sur le plan clinique dès lors que le patient réagit à des allergènes « majeurs » du pollen (ex. rôle de Bet v 4 chez un patient Bet v 1 positif ??)
L’absence de pertinence clinique d’une positivité pour les polcalcines semble assez bien établie. L’intérêt de cette famille d’allergènes (= plus exactement de protéines IgE-réactives) réside donc dans l’explication de positivités à des pollens qui ne concordent pas avec les données cliniques.
Il n’y a guère que le pollen de chénopode (Chenopodium album, Amaranthacées) qui, selon certains auteurs, recèle une polcalcine (Che a 3) qui, avec la profiline Che a 2, pourrait posséder une pertinence sur le plan clinique ![]() . Peut-être est-du à la forme particulière sous laquelle Che a 3 se présente : un tétramère
. Peut-être est-du à la forme particulière sous laquelle Che a 3 se présente : un tétramère ![]() plutôt qu’un dimère (Phl p 7
plutôt qu’un dimère (Phl p 7 ![]() ) ou un monomère (Bet v 4
) ou un monomère (Bet v 4 ![]() ) ?
) ?
La recherche d’une IgE-réactivité vis-à-vis d’un panallergène pollinique (profiline, polcalcine) a été suggérée pour guider la décision d’initier ou non une immuno-thérapie : ce point est discuté dans l’article concernant les profilines.



